19++ Alkane To Alkene Oxidation Or Reduction
Alkane To Alkene Oxidation Or Reduction. Oxidation and reduction of alkenes and alkynes. In a hydrogenation reaction, two hydrogen atoms are added across the double bond of an alkene, resulting in a saturated alkane.
 12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax From psu.pb.unizin.org
10.7 oxidation reactions of alkenes. Alkenes can easily be oxidized by potassium permanganate and other oxidizing agents. Oxidation, oxidative cleavage, and reduction reactions for alkenes and alkynes.need help with orgo?
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax
406 11 alkenes and alkynes ii. Internal alkene to epoxide intermediate. 2.) the solvent nh₃ supplies an h⁺ ion, forming a radical intermedatie. This video takes you through the various oxidation and reduction reactions you've covered back in alkene and alkyne reactions.
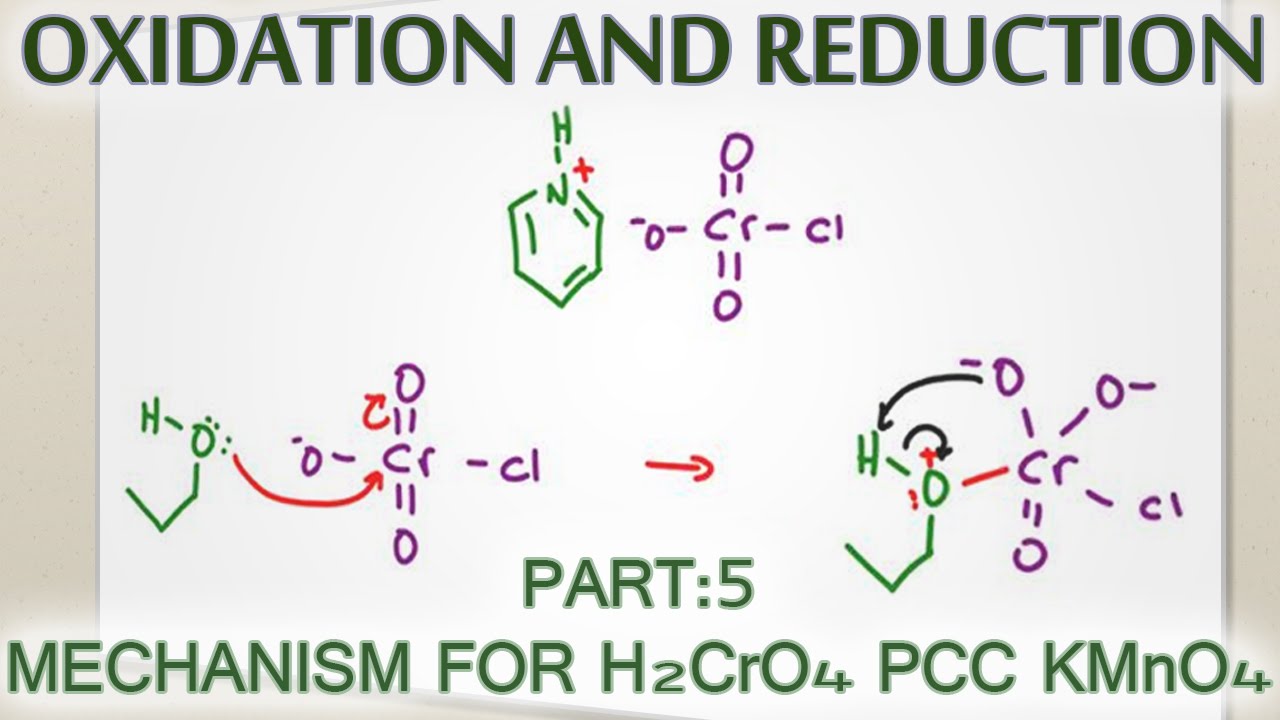 Source: youtube.com
Source: youtube.com
The selective oxidation of hydrocarbons is a challenging reaction for synthetic chemists, but common in nature. Acid and water (see above), but these simple addition conditions give rearrangements if a more stable carbocation is possible. At cold temperatures with low concentrations of oxidizing reagents, alkenes tend to form glycols. 3.) a carbanion is formed by the addition of an electron..
 Source: psu.pb.unizin.org
Source: psu.pb.unizin.org
This means that alkynes can be reduced by the addition of one or two equivalents of h 2, to alkenes and alkanes respectively: Internal alkene + mcpba/h3o+ epoxide then trans diol. This exothermic process is commonly used in the production of. Reduction of alkynes is a useful method for the stereoselective synthesis of disubstituted alkenes. The selective oxidation of hydrocarbons.