40+ Alkane To Alkene Reaction
Alkane To Alkene Reaction. At the same time, the electrons formerly making the bond between the two bromine atoms moves onto the other bromine, and the first bromine attacks the alkene with its lone pair. The alkenes can be halogenated by the addition of halogen across the double or triple bond in the same fashion as that of hydrogenation.
 Chapter 11 Organometallics, Part 5 of 5 Olefin From youtube.com
Chapter 11 Organometallics, Part 5 of 5 Olefin From youtube.com
This occurs via a cyclic bromonium or chloronium intermediate. This reaction goes through a two step mechanism, shown below. 1) addition of hydrogen halides 2) halogenation :
Chapter 11 Organometallics, Part 5 of 5 Olefin
The double bond is broken, and hydrogen is added to form alkane. Alkene + hydrogen → alkane this is called hydrogenation , and it needs a catalyst. There are four major types of addition reactions that can occur with alkenes, they include: The bromide ion quickly attacks the cationic center and yields the final product.
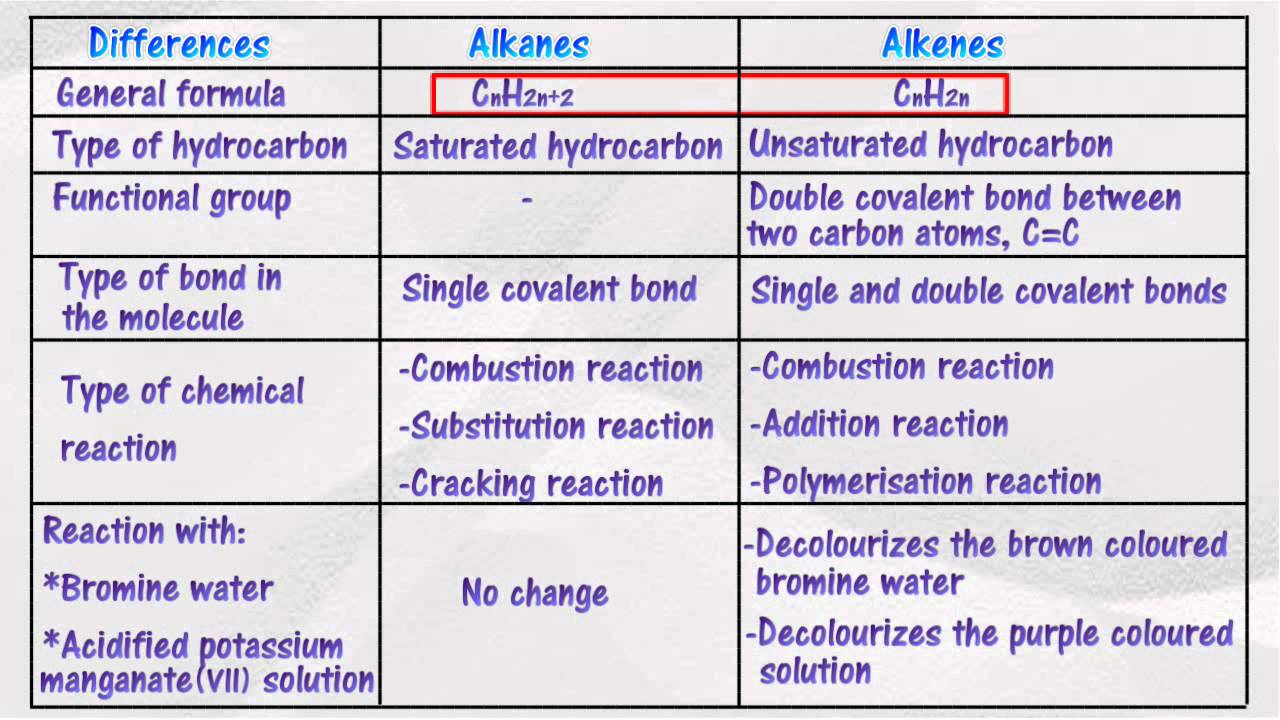 Source: youtube.com
Source: youtube.com
We can represent elimination reactions that form alkenes with the following general equation where a and b are atoms or groups of atoms. F 2 > cl 2 > br 2 > i 2.fluorination of alkanes is too vigorous to be controlled under normal conditions while iodination is very slow and a reversible reaction. Reactions of alkanes, alkenes, and cycloalkenes*.
 Source: pinterest.com
Source: pinterest.com
Reaction in which the elements of water (h and oh) are Reactions of alkanes, alkenes, and cycloalkenes* purpose: We can represent elimination reactions that form alkenes with the following general equation where a and b are atoms or groups of atoms. To investigate the physical properties, solubility, and density of some hydrocarbon. At the same time, the electrons formerly making.
 Source: youtube.com
Source: youtube.com
F 2 > cl 2 > br 2 > i 2.fluorination of alkanes is too vigorous to be controlled under normal conditions while iodination is very slow and a reversible reaction. A variety of different types of substrates undergo elimination reactions to form alkenes, but many of these reactions have common features. We can represent elimination reactions that form alkenes.
 Source: youtube.com
Source: youtube.com
We will review their nomenclature, and also learn about the vast possibility of reactions using alkenes and alkynes as starting materials. You would typically see something like sulfuric acid (h 2 so 4) as a catalyst in this reaction. • the catalysts is not soluble in the reaction media, thus this process For instance, hydrogen halides can be added to.
 Source: ppt-online.org
Source: ppt-online.org
For instance, hydrogen halides can be added to an alkyne by way of a mechanism similar to that of alkenes. The reaction of alkenes with the halogens cause the removal of the functional group of the alkene. View reactions of alkanes and alkenes hw.rtf from as 2 at northwood high school. The rate of reaction of alkanes with halogens follows.
 Source: youtube.com
Source: youtube.com
Alkene alkyne four major additions: The bromide ion quickly attacks the cationic center and yields the final product. To use physical and chemical properties to identify an unknown. For instance, hydrogen halides can be added to an alkyne by way of a mechanism similar to that of alkenes. At the same time, the electrons formerly making the bond between the.